The most reliable medicine for Alzheimer’s will certainly be obstructed for usage on the NHS on Wednesday.
Regulators are anticipated to proclaim the brand-new therapy for the condition risk-free for usage yet the rationing body for the health and wellness solution will quickly rule that it is as well pricey for NHS clients.
The choice is readied to let down charities and advocates that have actually asked for much better accessibility to freshly arising medications on the NHS.
Donanemab has actually been called game-changing and research study revealed it slowed down the development of Alzheimer’s by 35 percent. Scientists claimed it can indicate clients have the ability to live at home with a much better lifestyle for an additional 2 years.
The choice on donanemab– the 2nd medicine located to reduce the progression of Alzheimer’s condition– is readied to mirror one absorbed August when lecanemab, the initial development therapy for the problem, was accredited.
The draft support from the Medicines and Healthcare items Regulatory Agency (MHRA) will certainly indicate clients will just have the ability to acquire either medicine from personal centers unless they become part of medical tests. Health insurance plan are not likely to cover expenses.
Charities and pharmaceutical firms have actually criticised the National Institute for Health and Care Excellence (Nice)– the rationing body for the NHS– for neglecting the expenses birthed by households and culture, in making their analyses.
Almost 1 million individuals in the UK are dealing with mental deterioration, consisting of one in 6 individuals over the age of 80. The number is anticipated to get to 1.4 million by 2040 as Britain ages.


The substantial bulk of treatment is either supplied by enjoyed ones or spent for independently, yet Nice omits these “non-medical” expenses of treatment in their choice making.
Estimates recommend that the UK invests around ₤ 42 billion a year on mental deterioration, with the majority of the expenses birthed by households and social treatment bodies. Forecasts have actually claimed that this number can get to ₤ 90 billion by 2040.
The MHRA’s choice has actually been struck by hold-ups, with the regulatory authority at first preparing to phone in July– the very same time that it was authorized for usage in the United States.
Prof Sir John Hardy, the chairman of molecular biology of neurological condition at the UCL Institute of Neurology and among the globe’s leading scientists in the area, claimed he anticipated Nice to once more “come down on the wrong side of the argument” concerning medications which were “game-changing”.
The researcher was the initial to recognize the function of amyloid in Alzheimer’s condition which has actually currently brought about medications which function by removing the healthy protein.

Prof Hardy claimed: “These drugs can give people an extra two years at home, rather than in a nursing home. That is time enjoying their lives, having holidays, this is important stuff.”
The researcher– that explained that he has actually sought advice from for both Eisai– which makes lecanemab– and Lilly– that makes donanemab– claimed: “These are finely balanced arguments, but I do think they’ve come down on the wrong side of it.”
“I also think that the benefit of approval would be that it would kick NHS dementia care into shape – which really needs to happen. These drugs will come down the line at some point, and I don’t think the NHS is ready for them,” he claimed.
Lilly approximates the price of the medicine is $32,000 (₤ 24,600) in the United States, which has to do with 25 percent more than lecanemab.
But it has the benefit of being a 30-minute regular monthly intravenous shot, as opposed to an hour every 2 weeks, reducing the price of providing it for the NHS.
In the United States, complete therapy expenses for donanemab– consisting of surveillance and scans– ordinary $78,000 each year (₤ 60,000) per client.
Patients can likewise quit taking it if it properly removes the amyloid healthy protein in the mind that the medicine targets.
By comparison, lecanemab is provided forever, till condition gets to a modest phase.
In tests, donenamab slowed down cognitive decrease by 35 percent, making it a lot more reliable than lecanemab, which slowed down the condition by 27 percent.
Almost fifty percent of individuals on donanemab had no medical development of condition after a year, compared to 29 percent on sugar pill.
However, it has a greater threat of adverse effects, with the percentage of clients experiencing mind bleeds or swelling two times as high as its rival.
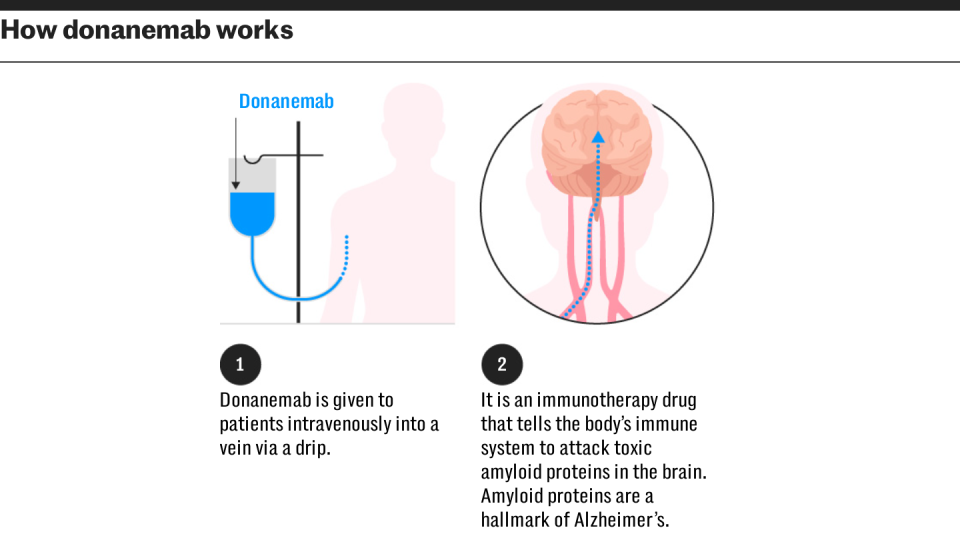
Both medications operate in a comparable method and are a type of monoclonal antibody, duplicating the activity of the body immune system to target certain healthy proteins, in this instance the elimination of amyloid from the mind.
Charities and pharmaceutical firms have actually claimed that clients will certainly lose out on the most effective brand-new therapies if the NHS does not essentially alter the method it determines the worth of medications.
Charity Alzheimer’s Research UK contacted Wes Streeting, the Health Secretary, in August and claimed his management was “needed to help enable fast and equitable access to a new generation of treatments”.
Chris Stokes, UK basic supervisor of Eli Lilly and Company, that makes donanemab, today prompted Nice to think about a therapy’s financial and social advantages in addition to the amount of “quality years” it can include in a client’s life-span.
These consist of whether taking a medicine can make it less complicated for a person to return to function, or lower their demand for carers.
“If we don’t incorporate the wider value into the discussion, then there is a real risk that patients will miss out on innovative treatments,” Mr Stokes informed The Sunday Times.
When lecanemab was declined in Nice’s draft support, the Alzheimer’s Society claimed households throughout the nation had actually been left in “uncertainty and confusion”.
Alzheimer’s Research UK claimed the failing to consist of the price of caring in the design was “fundamentally unjust” claiming “the way these assessments are being carried out is just not fit for purpose”.
Broaden your perspectives with prize-winning British journalism. Try The Telegraph totally free for 3 months with limitless accessibility to our prize-winning internet site, unique application, money-saving deals and even more.








